- Do you need help? Here Us:
- +91 98857 04874
- mathesis@mathesis.co.in
ADSORPTION SYSTEM
Adsorption operations exploit bound solids ability to preferentially concentrate specific substances from solutions (gaseous or liquid) onto their surfaces. Thus, by contacting fluids with such solids, the required objective of purification or separation is also achieved.
The extent of adsorption of a given scenario is reached once equilibrium is established between the adsorbent and its contacting resolution. In apply, adsorption performance is also powerfully influenced by the mass transfer of the species between the solution and therefore the} adsorbent surfaces and also the adsorption reaction rate. Technically, adsorption is, therefore, an equilibrium-diffusion-reaction method.
For liquid-phase adsorption
- De coloring, drying, or de gumming of petroleum product
- Removing of dissolved organic species from water provides
- Removing odor, taste, and color from water Flow
- Advanced treatment of waste water (domestic and industrial)
- De coloring of crude sugar syrup and vegetable oils
- Recovery and concentration of proteins, pharmaceuticals, and bio-compounds from dilute suspensions
- Bulk separation of paraffin and ISO-paraffin
For gas-phase adsorption
- Recovering organic solvent vapors
- Dehydration of gases
- Removing harmful agents and odor for personal protection
- Air separation
- Separating traditional paraffin from ISO-paraffin aromatics
- CO2 capture for addressing temperature change
Types Of Adsorption Systems :
Adsorption systems can be referred to as packed beds since the adsorbing material is packed together to filter fluid streams. Two different design implementations are fixed bed adsorbers and fluidized-bed adsorbers.
Fixed-bed :
adsorbers utilize a stationary adsorbent to filter streams. These systems are used in applications ranging from large industrial operations to remove harmful VOCs (volatile organic carbons) to small consumer uses as filters and gas masks.
Fluidized-bed :
adsorbers utilize a more complex moving (fluidized) adsorbent to filter streams. This is achieved by inducing a stream velocity high enough to suspend the adsorbent particles. Fluidization allows for continuous regeneration and a uniform temperature gradient, giving these systems the advantage of running continuously.
Adsorbants - Types :
- Activated alumina is an adsorbent made of aluminum oxide (Al2O3). It is used as a desiccant for drying gases and air and as a fluoride filter for drinking water.
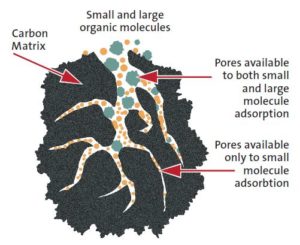
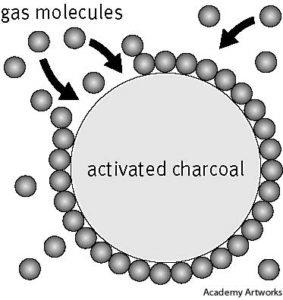
- Activated carbon is roasted organic material (coconut shell, bone, wood) that forms porous granules. It is a versatile and inexpensive adsorbent that comes in many sizes and has a range of applications from gas, water, and metal purification to air filtration. It is the most commonly used industrial adsorbent.
- Molecular sieves or zeolites are naturally occurring adsorbents with uniform pore size that can be tuned to be highly selective. They are used as dehumidifiers and air purifiers due to their high retention and adsorption capacities even at high temperatures.
- Silica gel or silicon dioxide is a common desiccant used in food preservation, humidity control, and various medical devices.
Specifications to be considered for Adsorption Systems Selection
- Selectivity is the amount of specificity an adsorbent has in the materials that it can capture.
- Capacity is the amount of contaminant that adsorption equipment can capture before the adsorbent is saturated and requires renewal.
- Regeneration is the ability of the adsorbent in the system to be reused. This is important for systems with large-volume or high-cost materials where iterative replacements would be expensive.
- Maximum working pressure
- Fill size
- Maximum liquid or gas flow rate
- Other factors that affect system design are the equipment’s pressure drop gradient or required operating power.

